What is the STAAR Study?
The STAAR Study will investigate the safety and tolerability of an investigational gene therapy called ST-920 in Fabry disease.
It is hoped that the investigational gene therapy ST-920, may result in an increase in functional α-Gal A enzyme, which is lacking in patients with Fabry disease.
The results from this study will add to the growing body of evidence and knowledge about gene therapy overall, and gene therapy to treat Fabry disease specifically. The data generated may help to develop future treatments for the benefit of more patients with the disease.

Who could be eligible to participate?
Men and women aged 18 years or older with a diagnosis of Fabry disease.
What will taking part involve?
Full details of the study will be described in detail with the study doctor during the next stage of your discussions about STAAR.
Overview of the STAAR study
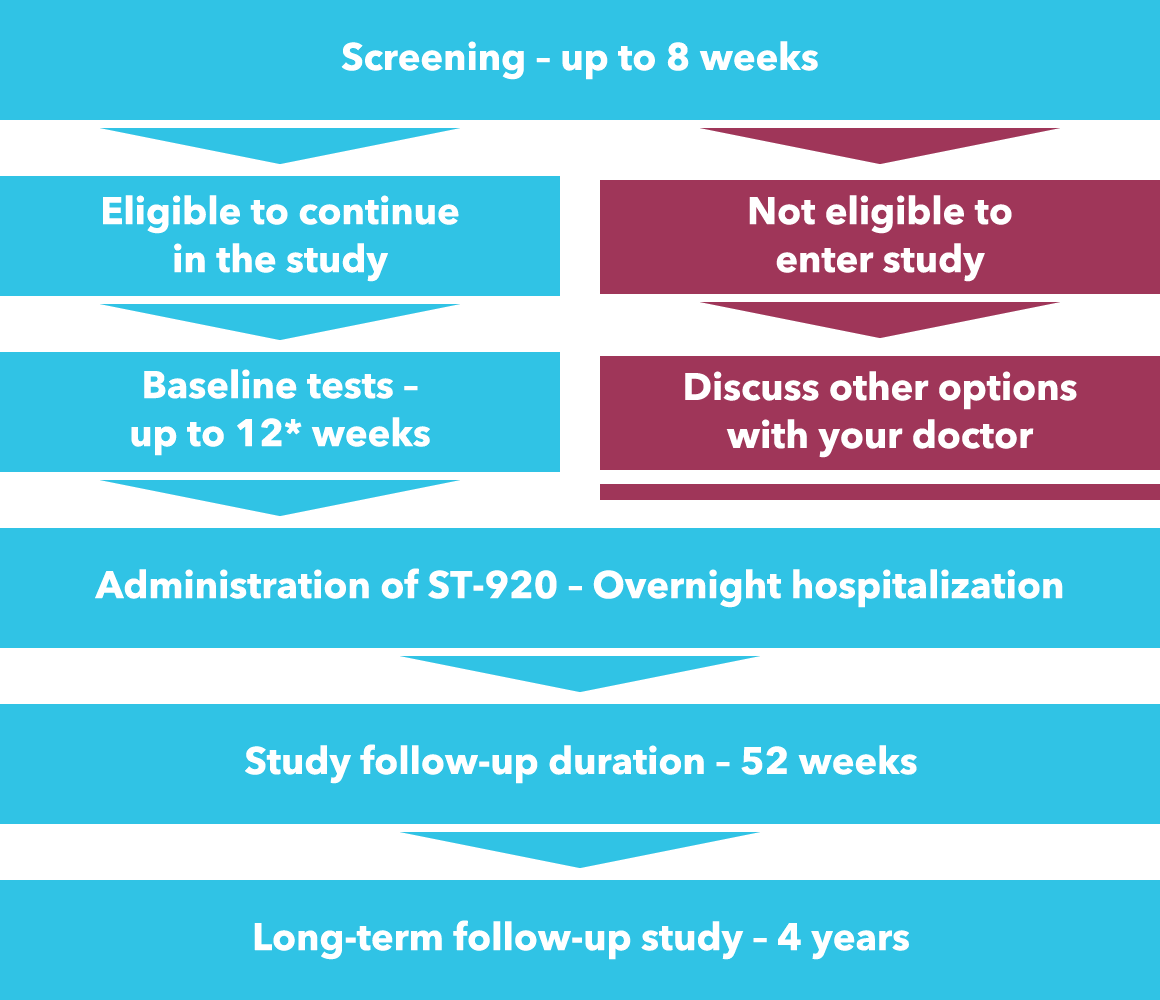
Screening stage – up to 8 weeks
Click here to check if you are suitable to enter the study. Screening involves medical tests and procedures including medical history, blood and urine samples, physical examination, COVID-19 assessment, pregnancy testing for women and tests to check how your heart, kidneys, liver and lungs are working. If blood tests show that you have any antibodies against the vehicle (AAV6) used in our investigational gene therapy ST-920, you will not be able to participate in this study. If you are eligible, you will have the opportunity to consent to continue onto the next stages of the study.
Baseline stage – up to 12* weeks
The baseline stage includes similar tests to those conducted during screening, COVID-19 assessment, as well as further tests to establish what is normal for you and completion of some questionnaires.
Administration of ST-920 – up to 2 days
- ST-920 is currently a one-time study treatment that will be given as an infusion into a vein in the arm (no further treatment with ST-920 will be given during the course of the study)
- You will come into hospital for the infusion. It will take between 2–8 hours to complete the infusion, depending on your body weight and dose
- You will stay in hospital for 24 hours or a little longer so we can monitor you closely, carry out observational tests and take blood and urine samples
- Blood tests will be performed twice weekly until week 20 to monitor liver function. If your liver tests show that you may be having an elevated immune response to ST-920, you may be given a medication called prednisone (or equivalent) to help treat any further immune response. This medication will be taken in gradually decreasing doses when liver enzymes have come back to acceptable levels
Please let your study doctor know if you will be receiving the COVID-19 vaccine, or any other vaccine, as this may affect when you receive your ST-920 infusion.
Follow-up after ST-920 administration – up to 52 weeks
- Your first visit back to see us will be about 6 days after you receive the infusion of ST-920
- Then, you will need to visit the study clinic every 2 weeks for the next 8 weeks
- If you are progressing well, the clinic visits are reduced to every 4 weeks until the end of the 52 week follow-up period
- Some tests can be completed at home, your study team will inform you of this
Entry into a long-term follow-up study – 4 years
After the 52 week study has ended, you can, and should, continue to be monitored in an additional 4-year long-term follow-up study. This is to monitor how you are doing and for any potential long-term side effects.
Potential study benefits and risks
It is possible that taking part in this study may improve the outcome of your Fabry disease, however this is not known. ST-920 may improve your symptoms, but there is also a chance that your symptoms may be no better, or worse, than they would have been if you had not received ST-920.
Potential benefits
Even if there is no benefit to you, the results will help to shape future scientific research that could result in better treatment success in the future for people with Fabry disease.
Medical advances depend on participation of people in clinical studies, and every participant in a study makes a positive contribution towards learning more about medicine and future medical treatments, particularly for rare diseases where fewer patients are available to help.
People who enter into clinical studies are contributing to the generation of data that may advance future treatments for the benefit of more patients.
The full potential benefits and risks of participating in the study will be described in detail with the study doctor during the next stage of your discussions about the STAAR Study.
Potential risks
All investigational medications carry a risk of side effects.
Unexpected and severe risks are possible in research studies like STAAR where genes have been modified, and have not previously been tested in humans.
ST-920 is a once-only infusion that cannot be reversed. It is currently not known if ST-920 will increase α-Gal A enzyme in the blood, or if there will be any side effects in humans, as this is the first time that it will be studied in people. Therefore, people in STAAR will be closely monitored for any side effects. In addition, participants may become ineligible to other gene therapies using a similar vehicule due to exposure to ST-920.
For more details on the study, visit clinicaltrials.gov
*May be extended to 24 weeks in case of delays due to COVID-19
